CONTACT
We will reply as soon as possible.
Enevia Health, LLC
30 N Gould Ste N, Sheridan, WY 82801, USA
By Natalia Marble, Co-founder of Enevia Health
In this interview we are going to talk about a compound that many of you have heard about, it is the OSR/EMERAMIDE or NBMI. This compound is a heavy metal chelator with amazing properties that has the AUTIST community very interested. To talk about it, we have invited Dr. Haley Boyd, the person who invented this compound.
1. What is the OSR?
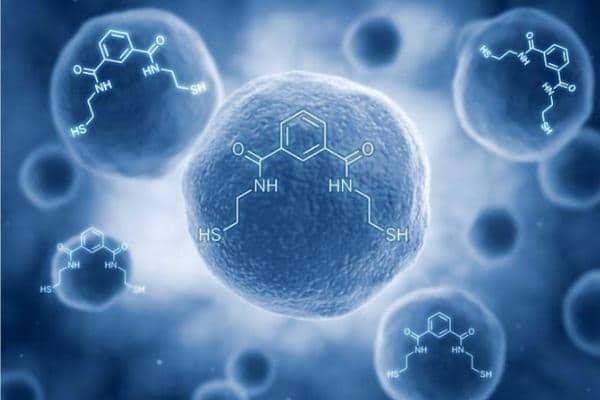
OSR (Oxidative Stress Relief) is now called emeramide and is being studied for marketing approval as an FDA/EMA-approved drug to treat mercury toxicity. In scientific papers it is called NBMI which is the abbreviation of its complex scientific name.
2. What was your goal when you formulated OSR?
The initial development of the OSR was based on various aspects of the research I have conducted throughout my life. The highlight was my belief that the rise in autism in the US correlated with the DC-USA mandated immunization program. Thimerosal-preserved vaccines were used in infants and infants had exposure to mercury through the preservative. A publication from a medical group in Toronto, Canada, revealed that a group of babies with umbilical cord infections were treated with a topical application of a commonly used antiseptic containing thimerosal, 10 babies died of mercury toxicity. Babies apparently can't handle exposure to thimerosal like teenagers and it accumulates in their tissues to very toxic levels. The FDA subsequently banned the sale of thimerosal-containing topical antiseptics following this report, but the CDC allowed their use to preserve certain infant vaccines. This later led to the reduction or elimination of the use of thimerosal as a preservative in childhood vaccines in the US but even today, it apparently is not banned.
3. Did you achieve what you intended?
He had NIH funding to study nucleotide-binding proteins in the brain of the Alzheimer disease (EA) since we had published results showing major differences using bioprobes that he had invented earlier. Following this lead, we publish data showing that mercury, and mercury alone, when added to normal brain tissues can easily replicate the abnormal properties of microtubulin found only in brain tissues in Alzheimer's disease. Therefore, he was very interested in any compound that could fix mercury (Hg2+) and eliminate or reduce its toxicity. I was chair of the chemistry department at the time and hired an assistant professor interested in developing treatments for heavy metal toxicity in coal mining water. I attended his presentations to graduate students about possible chelators to treat such water. I was very interested in a compound that later failed mainly because it was not soluble in water but had a very high affinity for various toxic metals, this being OSR (also known as NBMI, emeramide).
In my opinion the current chelators used in medicine (DMSA, DMPS, EDTA, etc.) were not effective because they were charged hydrophilic compounds that do not effectively cross the biomembranes of normal cells, nor the blood-brain barrier to enter the central nervous system. Furthermore, they were not true chemical chelators as the two thiols present in each have adjacent carbons and are brought together to allow the mercury to sterically fit together, thus forming sandwich complexes with mercury that are weaker bonds.

4. What are the qualities of the OSR, can you explain them please?
What is the comparison between commonly used chelators and OSR?
The commonly used mercury chelators DMPS and DMSA are charged and do not effectively enter cells. They are excreted rapidly, within 4 to 8 hours in the urine with or without bound metals. The only toxic metals that are eliminated are those found inside the arterial walls, they do not reach the intracellular metals that cause toxicity.


5. About 3,000,000 packets of OSR were sold in the US as a dietary supplement, with no reported side effects. What happened? Why did you stop selling it?
I introduced OSR as an antioxidant dietary supplement, at the time under the FDA ruling that a dietary supplement could be any natural product or a combination of two natural substances. The OSR was the combination of dihydroxybenzoate (found in various berries) and cysteamine (found in meat and at the terminal end of coenzyme-a). Benzoates are commonly regarded as GRAS (generally recognized as safe food additives) compounds and cysteamine is a breakdown product of cysteine (it is the decarboxylated amino acid cysteine). I initially developed OSR because it was a structure that had two important structural features that allowed it to: (1) bind very strongly to toxic metals attracted by thiols, and (2) be uncharged and able to pass through biomembranes to reach intracellular cytosols.

We had tested the OSR compound and knew that it bound strongly to various toxic metals, we are continuing to test it to show that ingesting or injecting OSR causes very few, if any, toxic effects! Then we test OSR for ORAC (Oxygen Radical Absorbance Capacity) and we found it to be excellent in this regard. We sent it to a commercial ORAC testing company and confirmed this property. There will be few, if any, antioxidants that can be taken orally and meet the ORAC score of the OSR. Knowing that autistic children suffered from oxidative stress and many were treated with ineffective antioxidants, we thought that OSR would be a good resource as OSR is safe, enters all body tissues, is a powerful antioxidant and likely chelates any toxic metals. found on the body. It eliminates toxicity by binding non-free Fe3+ which donates an electron in the production of hydroxyl free radicals in the fenton reaction. After submitting OSR as a dietary supplement to the FDA, we gave samples to physicians who had autistic children to use and review. Some physicians reported that the porphyrin profile returned to normal. Next, we introduced OSR commercially as a dietary antioxidant.
*** Clarification from Dr. Haley on why they stopped marketing OSR in the US:
The short answer is that the FDA after two years of marketing OSR with no "related adverse effects" reported to EmeraMed, nor to the FDA, sent us a letter saying that since OSR was being used to treat a medical condition it had to be considered an unapproved drug and not a food supplement.
The medical condition was autism, as noted in blog site reports of parents of autistic children reporting improvements in their children's health from taking OSR. I don't know who told the FDA about these sites, but they weren't directly associated with EmeraMed and our company didn't make such claims because we knew it would lead to the FDA stopping their use.
However, the FDA in its withdrawal letter made its position clear, any compound sold that had a positive effect on any disease had to be classified as a drug and had to have FDA approval. Since we did not have FDA drug approval for OSR, the FDA sent its agents to our company daily for a week to make sure we stopped sending OSR to doctors and dentists, and therefore parents of autistic children.
I contacted an experienced attorney to deal with the matter and he told me that if I hired him, he was sure I could win my case. However, he also told me that, in his opinion, it would cost more than a million dollars and that in the end, based on his experience, the FDA would promptly seize all of the lab and computer equipment, and bring other charges against EmeraMed and the affiliates who helped us produce the compound. He recommended that I take the OSR through the drug approval as it was very safe and effective and I obviously needed it. So that's what I did.
Given our research, we prepare and apply the ODR (Orphan Drug Requests) for both the EMA and the FDA and both were accepted. These ODRs were for toxic heavy metal treatment based primarily on the prevention and treatment of mercury toxicity.
We currently have OSR (such as Emeramide) submitted to the FDA as a drug to treat mercury toxicity. We have met twice with FDA committees to discuss what else is needed to produce an acceptable New Drug Application (NDA) that could lead to marketing authorization. The FDA has made statements to date accepting that Emeramide (OSR) is safe and effective based on our animal studies. They also agreed that Emeramide is safe for human use due to two years of marketing and 5 Phase I/II human trials conducted with no significant drug-related adverse effects.
However, the FDA, which originally recommended that Emeramide be approved under the FDA's animal rule, changed its mind. The FDA's new position is that EmeraMed would have to conduct Phase II and III human trials to demonstrate its efficacy in humans.
However, when we asked the FDA to identify a mercury-toxic human population in the US that could serve as test subjects for such Phase III studies, they have yet to respond. It is rare in the US for a physician to suggest that mercury toxicity is in any causal way involved in any disease, including autism. Dentists who have made comments that dental amalgam exposes patients to toxic levels of mercury have been subject to action by the state dental board.
Physicians in the US mostly avoid outright diagnosing mercury exposure as the cause of any disease. I haven't heard of any clinics in the US that treat mercury toxicity. I honestly believe that our FDA does not consider mercury toxicity in the US to be a medical concern, even though they have stated on their website that dental amalgam is not safe for certain citizens.
I would like to point out that the FDA is not very proactive in developing drugs based on need. For example, I have not been contacted by anyone from the FDA about the very positive claims that parents of autistic children make on blogs, stating that the use of OSR produced the most promising positive effects in treating these children. This is amazing, since the rise of autism after the start of the CDC's mandated vaccination program around 1988 has been portrayed as a medical problem, and apparently still is in many countries. The FDA/CDC recommendation to remove thimerosal (ethylmercury) as a preservative from childhood vaccines shows that these agencies are aware of parents' concerns about preservatives in vaccines.
Finally, say that the safety of childhood vaccines is different in each country. Thimerosal-containing vaccines are not banned in the US, it's just that thimerosal-free childhood vaccines are available. It is likely that each nation has approached the safety of thimerosal-containing vaccines differently and this needs to be verified in each country.

6. AUTISM CURE… Let’s talk about autistic families who tried OSR we know that heavy metal toxicity is a big problem in this community, children who cannot properly detox accumulate these metals causing a variety of symptoms.
What these parents need to understand is that oxidative stress in their children began at a very young age, most likely caused by a toxin that induces the displacement of Fe3+ from normal tissue, leading to the formation of free radicals of iron and unbound hydroxyl and oxidative stress. Some autistic children will be more genetically susceptible than others.
7. What did families with autistic children tell you after taking OSR?
8. OSR ANALYSIS. We know that OSR excretion is through the stool, which makes the stool test the obvious choice. Are any of the metals excreted in the urine?
Once OSR binds to a cation with a +2 charge, the complex is neutral and is easily eliminated by the kidneys in the urine. The OSR-Hg complex formed within a cell has to be modified by the body's p-450 detoxification system to attach a charged water-soluble phase to the uncharged OSR-Hg complex to make it water-soluble and excretable. In phase I of the p-450 detox system a class of enzymes called CYP enzymes oxidize insoluble (hydrophobic) compounds preparing for adjuncts in the phase II modification of p-450 where the oxidized site added by CYP oxidation reacts with a phase II enzyme and loaded water-soluble component are attached. In the case of the OSR-Hg compound, this appears to be glutathione attached by the enzyme p-450 phase II GST (glutathione-s-transferase) that produces the glutathione-OSR-Hg complex that is soluble and excretable in plasma and is placed in the bile through the biliary transport system and then in the fecal matter. Mercury 80-90% is excreted by healthy humans in the feces.
This is how the glutathione-Hg-glutathione complex removes mercury through fecal matter in.
Do not miss the interview on the Enevia Health YouTube channel
OTHER QUESTIONS:
* How often would you test?
This must be determined by the doctor since all cases are different.
* Does OSR deplete minerals?
Emeramed has never seen burnout in our Phase I and II human studies. It has also not occurred in test animals unless exceptionally high doses are given over long periods of time.
* Are there any supplements that should or are recommended to be taken with the OSR?
Yes, a molybdenum supplement and ensuring adequate zinc levels would be reasonable.
* INGESTION Is it better on an empty stomach or with food? best taken with coconut oil?
OSR appears to be slightly better absorbed with a fatty meal.
* Morning or night?
No difference in absorption.
* How does OSR interact with stomach acid?
It doesn't seem to interact.
* Can it increase/decrease the pH of the stomach?
Not chemically since the pH of the protons in the OSR-thiols to be released has to be greater than 10.0
* Is it known where the OSR is mainly absorbed?
OSR peaks in plasma around 2 to 3 hours after ingestion. OSR enters the different organs from the plasma and does not appear to leave the organs (the liver and kidneys) to enter other tissues. This happens with or without mercury present in the tissues.
* Is it important not to take minerals with OSR? How long should intake of other minerals be separated from OSR to be safe?
It is very safe to take minerals while taking OSR but a minimum of 5-6 hours should elapse between the two intakes.
* Do you think taking OSR can increase adrenal stress? Is it recommended to support the adrenal glands? Do you remember any family with ASD talking about this topic?
I have no record of this, we have not studied it.
* Has anyone reported children being more anxious or waking up in the middle of the night when taking OSR?
No, on the contrary, the reports were that the children slept better. However, in my opinion this has not been properly studied.
* If it ever happened, did you recommend the parents to continue the doses and/or lower them and support the adrenal glands?
I have never broached this issue with a parent.

*Let's talk about sulfur/sulfur sensitivities, probably the only contraindication to OSR is that sensitivity and many ASD people have it, so I would NOT recommend taking OSR? Is there a way that people who have this sensitivity can take OSR?
Yes, sensitivity is eliminated with molybdenum supplementation, so taking OSR will be helpful.
* When is it advisable to take molybdenum?
As a supplement on a regular basis weeks before OSR treatment.
* What would be important for a parent of an ASD child to consider before giving OSR?
This must be decided by the doctor, but for example genetics, p450, mineral levels etc…
* What are the doses that people with ASD took and saw better results?
Between 100 to 400 mg/day. Larger and older children should take larger doses, but this should be done on the doctor's advice.
* Is it better to start with really small amounts and increase over time or is it better to use the full dose?
I always recommend going slow, but try to increase the dosage to an effective level within two weeks.
* What heavy metal will not join OSR?
We can assure that the OSR does not bind Ca2+, Mg2+, k+ and Na+ but we have not studied other metals to determine its binding.

* STORAGE, What is the best way to store OSR?:
Best kept refrigerated, but is reasonably stable at room temperature if kept sealed.
* How long can it be kept?
We have established a shelf life of about 5 years for emeramide. We have also tested OSR for 8-10 years and it was still good.
Can you tell us what the status of OSR is in the US?
It is not FDA-approved for any treatment and is only available through foreign chemical companies that must state that it is not for human consumption. As you know, there is an active black market for the sale of OSR in the US that our government makes no attempt to prevent. Emeramed does not sell OSR or emeramide.
* Is it possible to manufacture and sell OSR in Europe as a nutritional supplement?
I really don't know, it will depend on the country.
* What happened to compassionate use in the EU?
Emeramed shut it down because it was costing the company too much and not helping with regulatory approvals.
* When do you think you will get FDA approval to sell OSR again in the US?
I really do not know. We are going to test it but we are also trying to get approval in Latin America and Africa. This is not an easy business and it is full of companies that do not make a pure and proven safe product. I want to keep the use of OSR as safe as possible and I am concerned about the safety of copied products.
* The company that is creating OSR is based in Sweden. Why is it not a US based company?
Is not true. Emeramed is based in Ireland, with offices in Sweden and the US We have applied for and won Orphan Drug Development in the EU and US.
* Does OSR bind to Mg transiently and can it be used to enhance absorption into the body and cell membranes?
I have no indication of this happening.
* Would it be possible to make liposomal OSR? That is, surround it with a bubble of phosphatidylcholine?
Why? It is already hydrophobic to begin with and is easily absorbed into all organs of the body.
* Is it possible that the OSR joins the Free Iron and then finds Free Mercury and loses its link to the Free Iron? Will this happen with other heavy metals for example, Mercury could expel the lead and uranium together?
This is possible but highly unlikely as free mercury levels are incredibly low in the body.
* Some people need to take molybdenum to better tolerate OSR, is it possible that the thiols on the benzene ring of OSR degrade to sulfites, hence the need for molybdenum?
Thiols on the benzene ring are only converted to sulfites by reaction with hydroxyl free radicals in solution. If stored as a dry powder, OSR thiols are stable for years.
* Mercury and heavy metals damage heme and cytochromes, after the use of OSR, would it be necessary to replenish and help the body build these cytochromes using iron, copper and 5-AL-5-aminolevulinic acid?
They may be good co-treatments but more research is needed.
* If my child is sick (with a cold/virus), would you give them the OSR? We have heard you say in other interviews that OSR has antiviral properties, can you explain this please?
Absolutely, we know that OSR treatment raises intracellular reduced glutathione levels in mammals and that increased glutathione levels inhibit viral infection rates. This is why covid did not cause as many major problems in the younger population (higher glutathione levels) than in the older population (with much lower glutathione levels).

Youtube: https://www.youtube.com/c/EneviaHealthSL
Facebook: https://www.facebook.com/eneviahealth
Instagram: @enevia_health
We will reply as soon as possible.
30 N Gould Ste N, Sheridan, WY 82801, USA
Our groups are the ideal platform to learn and share your scientific concerns about neurodevelopment issues
*Our purpose is informational only, it is not intended to be a substitute for medical advice, diagnosis or treatment.
We are working on our website. For any queries, you can contact our customer service team at atencionalcliente@eneviahealth.com