CONTACT
We will reply as soon as possible.
Enevia Health, LLC
30 N Gould Ste N, Sheridan, WY 82801, USA
Primary immunodeficiencies (PID) are a series of genetic diseases in which there is a quantitative or functional alteration of different mechanisms involved in the immune response, which may entail, depending on the immunodeficiency, an increased risk of infections, immune dysregulation, autoimmune phenomena. , autoinflammation and neoplasms.
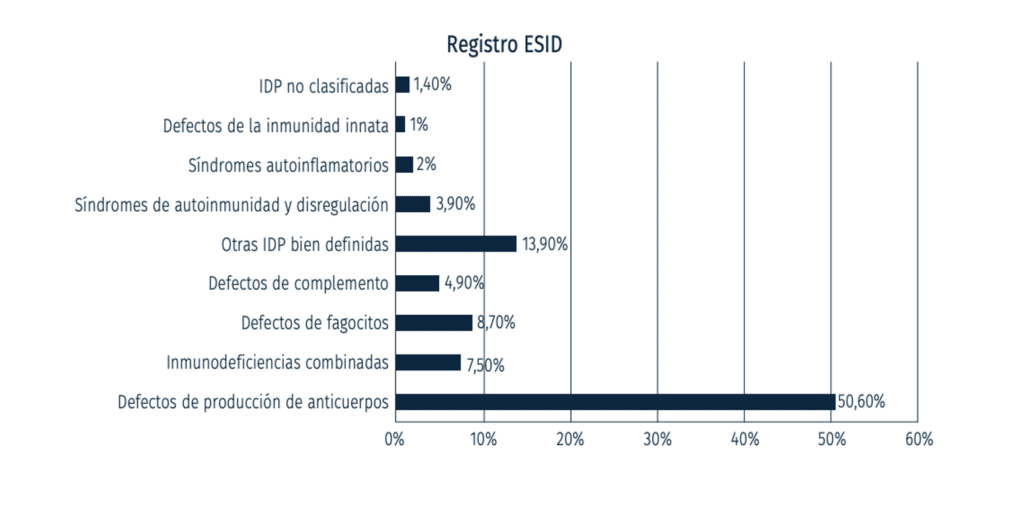
IDAP is the most common form of primary immunodeficiency in adults, accounting for about 10% of all primary immunodeficiency cases.
Specific immunodeficiency of polysaccharide antibodies (IDAP) is a condition in which the immune system does not produce enough antibodies against specific polysaccharide antigens (meaning proteins or polysaccharides that are part of bacteria, viruses, and other microorganisms), such as those found in some vaccines, such as pneumococcal vaccine, those with IDAP do not produce antibodies when receiving the vaccine.
In people with IDAP, the ability of the immune system to respond to specific polysaccharide antigens may be decreased or absent, increasing the risk of recurrent infections with encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae. These infections can be serious and can increase the risk of complications and mortality.
IDAP is a specific form of primary immunodeficiency and can be inherited or acquired. Genetic causes of IDAP may include mutations in genes that code for key proteins in the immune response, while acquired causes may include autoimmune diseases, chronic infections, treatment with certain medications, and other environmental factors.
Did you know that recurrent ear infections, respiratory infections, and recurrent skin infections can be signs of PRIMARY IMMUNODEFICIENCY OF POLYSACCHARIDE ANTIBODIES?
Mutations in some genes have been shown to be associated with specific immunodeficiency antipolysaccharide antibodies (IDAP), mutations in TACI, CD19, BAFFR, BLNK, ICOS, LRBA, NFKB1, and PIK3CD have been linked to IDAP and are considered pathologic. in this context. However, not all mutations in these genes necessarily cause IDAP, and may also be associated with other immunological diseases or have no significant effect on immune function.
Several genes have been identified that may be related to the condition. Some of the genes that have been associated with IDAP include:
TACI (Transmembrane Activator and CAML Interactor): This gene is involved in the regulation of antibody production and has been associated with reduced production of specific antibodies against polysaccharide antigens. (1)
The c.446C>T mutation in exon 4 of TACI has been found to produce a truncated protein lacking the transmembrane region and associated with IDAP.
CD19 (Cluster of Differentiation 19): This gene encodes a protein found on the surface of B cells and is involved in B cell activation. Mutations in this gene have been associated with reduced production of specific antibodies against polysaccharide antigens.(2)
The c.823T>C mutation in exon 6 of CD19 produces a truncated protein that is associated with IDAP.
BAFFR (B-cell Activating Factor Receptor): This gene encodes a receptor found on the surface of B cells and is involved in B cell survival and activation. Mutations in this gene have been associated with reduced production of specific antibodies against polysaccharide antigens. (3)
the c.250C>T mutation in BAFFR exon 3 produces a truncated protein that is associated with IDAP.
BLNK (B-cell linker protein): This gene codes for a protein found in B cells and is involved in the transmission of signals for the production of antibodies. Mutations in this gene have been associated with reduced production of specific antibodies against polysaccharide antigens. (4)
ICOS (Inducible T-cell costimulator): This gene encodes a protein found on the surface of T cells and is involved in T cell activation and antibody production. Mutations in this gene have been associated with reduced production of specific antibodies against polysaccharide antigens. (5)
The c.309G>A mutation in exon 3 of ICOS produces a protein with an alteration in the ligand-binding region that is associated with IDAP.
LRBA (LPS-responsive beige-like anchor protein): This gene codes for a protein found in immune cells and is involved in the regulation of the immune response. Mutations in this gene have been linked to various immunological conditions, including IDAP. (6)
the c.4723C>T mutation in exon 45 of the LRBA produces a truncated protein that is associated with IDAP.
NFKB1 (Nuclear factor kappa B subunit 1): This gene encodes a protein that is involved in the regulation of the immune response. Mutations in this gene have been associated with reduced production of specific antibodies against polysaccharide antigens. (7)
The c.1558C>T mutation in exon 13 of NFKB1 produces a protein with an alteration in the DNA-binding region that is associated with IDAP.
PIK3CD (Phosphatidylinositol 3-kinase delta): This gene encodes a protein that is involved in regulating immune cell function. Mutations in this gene have been linked to various immunological conditions, including IDAP. (8)
The c.3061A>G mutation in exon 24 of PIK3CD produces a protein with a kinase domain alteration that is associated with IDAP.
It is important to remember that you can have primary immunodeficiency without having the aforementioned genes affected..
Some of the symptoms that people with IDAP may experience include:
Ear infections: Patients with IDAP may experience recurrent ear infections, which can cause pain, fever, and temporary hearing loss.
Recurrent respiratory infections: People with IDAP are at increased risk of developing recurrent respiratory infections, such as sinusitis, pharyngitis, bronchitis, and pneumonia.
Infections of the skin and mucous membranes: People with IDAP may have an increased susceptibility to infections of the skin and mucous membranes, such as cellulitis, impetigo, and conjunctivitis.
systemic infections: In severe and untreated cases of IDAP, systemic infections such as sepsis and meningitis may occur.
1. Measurement of serum levels of immunoglobulin G (IgG), IgM and IgA: Patients with specific immunodeficiency of antipolysaccharide antibodies may have low levels of specific IgG and IgM against polysaccharide antigens. Therefore, measurement of serum levels of these immunoglobulins can help identify immunodeficiency.
2. Measurement of Specific Antibody Levels: ELISA or EIA (enzyme-linked immunosorbent assay) tests are used to measure specific levels of antibodies against polysaccharide antigens. Patients with specific immunodeficiency for antipolysaccharide antibodies may have low levels of specific antibodies against these antigens.
3.B cell function tests: Tests of B-cell function, such as B-cell count and determination of B-cell proliferative capacity, can help to assess B-cell function in patients with specific immunodeficiency of antipolysaccharide antibodies.
4.Pneumococcal vaccine test: Administration of pneumococcal vaccine can help assess the patient's response to polysaccharide antigens. Patients with specific immunodeficiency for antipolysaccharide antibodies may have a poor response to the vaccine, which may indicate immunodeficiency.
The pneumococcal vaccine test is performed by administering the pneumococcal vaccine and evaluating the patient's response to the polysaccharide antigens present in the vaccine. The pneumococcal vaccine is a vaccine that contains a combination of polysaccharides from different strains of the Streptococcus pneumoniae bacterium, which is a common cause of pneumonia, meningitis, and other infections.
The pneumococcal vaccine is administered by intramuscular or subcutaneous injection into the patient's arm. Following administration of the vaccine, the patient's immune system is expected to produce specific antibodies against the polysaccharide antigens present in the vaccine.
Serological tests can be performed to measure the levels of antibodies against the polysaccharide antigens present in the vaccine in the patient's serum before and after the administration of the vaccine. Antibody levels are measured by enzyme-linked immunosorbent assay (ELISA) or agglutination assay.
The response to the vaccine is assessed by comparing the antibody levels before and after the administration of the vaccine. In general, antibody levels are expected to increase significantly after administration of the vaccine. If antibody levels do not rise adequately, it may indicate a specific immunodeficiency of antipolysaccharide antibodies.
It is important to evaluate the total immunoglobulin levels, especially the IgG2 and IgG3 subclasses. If the count is low, caution is recommended when administering the pneumococcal booster dose. There are specialists who recommend a protocol drug to attenuate the immune response to the vaccine.
At Enevia we offer you the opportunity to test immunoglobulins in different laboratories in Spain at the best prices, check our website http://www.eneviahealth.com
It is important to note that IDAP is often misdiagnosed or underestimated as patients may have recurrent infections that are treated without accurate diagnosis. Therefore, greater knowledge and clinical awareness of IDAP is required for its proper diagnosis and treatment.
At Enevia Health we are working to be able to offer the possibility of doing the pneumococcal vaccine test.
(1) TACI:
(2) CD19:
(3) BAFFR:
(4)BLNK:
(5) ICOs:
(6) LRBA:
Lopez-Herrera G, Tampella G, Pan-Hammarström Q, Herholz P, Trujillo-Vargas CM, Phadwal K, Simon AK, Moutschen M, Etzioni A, Mory A, Srugo I, Melamed D, Hultenby K, Liu C, Baronio M , Vitali M, Philippet P, Dideberg V, Aghamohammadi A, Rezaei N, Enright V, Du L, Salzer U, Eibel H, Pfeifer D, Veelken H, Stauss H, Lougaris V, Plebani A, Gertz EM, Schäffer AA, Hammarström L, Grimbacher B. Deleter
We will reply as soon as possible.
30 N Gould Ste N, Sheridan, WY 82801, USA
Our groups are the ideal platform to learn and share your scientific concerns about neurodevelopment issues
*Our purpose is informational only, it is not intended to be a substitute for medical advice, diagnosis or treatment.
We are working on our website. For any queries, you can contact our customer service team at atencionalcliente@eneviahealth.com