CONTACT
We will reply as soon as possible.
Enevia Health, LLC
30 N Gould Ste N, Sheridan, WY 82801, USA
The growing knowledge about the intestinal microbiota, the importance of the initial composition and the recognition of early childhood as a key time window in the establishment of the microbiota and its influence on the programming and development of the immune and nervous systems, lead to the fact that today There are multiple pathologies linked to intestinal dysbiosis and hyper-permeable intestine (leaky gut). Specifically, the study of the gut-brain axis and its impact on both autism spectrum disorder (ASD) and other neurological and psychiatric pathologies, lead us to recognize diet and lifestyle as important factors involved in the pathogenesis and pathophysiology of autism. the same. Therefore, nutritional intervention becomes relevant as an associated therapeutic approach, since changes in diet quickly impact the microbiota. The nutrient exclusion diet (gluten, casein, and soy free, low in sugar, as well as supplementation of deficient nutrients) continues to report improvement in gastrointestinal, neurological, and behavioral symptoms, as well as sleep disorders and hyperactivity in some patients.
The microbiota of the human body is made up of more than 1014 microorganisms present in different areas of the body. The gastrointestinal tract constitutes the largest host interface exposed to the external environment and contains approximately two thirds of the human commensal microbial community. Although the gut microbiota (IM) includes viruses, fungi, protozoa, archaea, and bacteria, the bacterial component is the most studied. The main bacterial phyla of IM include Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. The increasing use of high-throughput deep sequencing technology over the past decade has revealed that the gut microbiome encodes 3.3 million genes, 100 times the number of human genes. For this reason, the gut microbiome is also called the "second human genome."
The components of MI are divided into three groups according to their functions: beneficial commensal microorganisms, potentially sensitive pathogens, and pathogenic bacteria. “Beneficial” commensal microorganisms maintain a healthy environment in the host and offer benefits, as well as interacting with host tissues in a cooperative and non-pathogenic manner. We call this “Eubiosis”. Taxonomic studies show that even in homogeneous human populations, there are few common bacteria, and there is even individual variability on different days, so the functional and metabolic capacity of the microbiota becomes more relevant. The novel concept of "holobiont" includes the human being and the microbiota that inhabits it.
There are multiple pathologies today linked to intestinal dysbiosis and hyper-permeable intestine (leaky gut). Advances in development, associated with the reduction of costs for research in different pathologies, have made it possible to identify new "specific intestine-organ axes", of which the Gut-Brain Axis is the most studied.
The establishment and development of a beneficial composition of the microbiota occurs during the first 1000 days of life. This time window is crucial in the programming and development of the immune and nervous systems. Environmental and parental factors such as host genetics, mental health, nutrition, mode of birth (delivery/cesarean section) and diet, antibiotic exposure, immune activation, and prenatal microbiota composition can modulate the composition of the microbiota of the mother and the baby. Of all these factors, nutrition in early life plays a fundamental role in perinatal programming and in modulating the microbiota of the offspring. Disturbances at this critical stage have long-lasting effects on the microbiota-gut-brain axis.
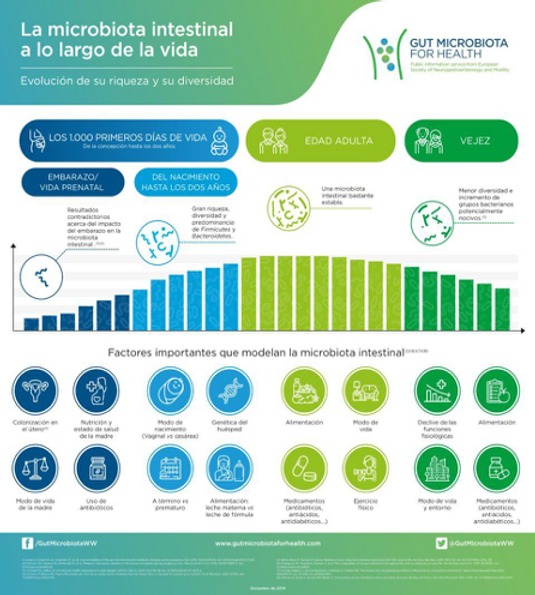
According to clinical findings, inflammation and dysfunction of the immune system mediated by the composition of the microbiota are key elements in the development of gastrointestinal problems and other extra-intestinal diseases such as Autism Spectrum Disorder (ASD). Dysbiosis in these children has been related not only to the usually comorbid gastrointestinal problem, but also to the intensity of autistic symptoms, suggesting that bacterial activity and the resulting metabolites are involved in the development and severity of ASD. These findings are the basis for the “microbiome-gut-brain axis” concept, which posits that there is a two-way interaction between gut bacteria and the brain. Although it is known that this communication occurs through hormones and neurotransmitters released by the intestinal endocrine system depending on the microbiota and metabolites that exist, the specific underlying mechanisms of this physiological interaction are not entirely clear and more research is needed to gain a better understanding of the physiological communication between the gut and the brain. Furthermore, the direction of causality is still being studied, that is, whether alterations in the microbiota cause inflammation and imbalances in the immune system or vice versa.
However, compared to neurotypical children, children with ASD show a higher abundance of Bacteroidetes (Bacteroides and Parabacteroides) and some genera of the Firmicutes phylum (specifically Clostridium, Faecalibacterium and Phascolarctobacterium) along with a lower abundance of Coprococcus and Bifidobacteria. Spore-forming bacteria, such as Clostridium, release pro-inflammatory toxins that can reach the brain through the blood and have been associated with repetitive behaviors and gastrointestinal disturbances in ASD. On the other hand, short-chain fatty acids (SCFA) participate in the proper functioning of the intestinal immune system by modulating gene expression. Any imbalance in its concentration can alter intestinal homeostasis and trigger peripheral inflammation. These also reach the brain through the blood and influence its development by modulating the production of serotonin and dopamine. In this sense, propionic acid (PPA), produced mainly by Bacteroidetes, is one of the main neurotoxic SCFAs when its concentration increases. Faecalibacterium is related to the regulation of interferon (IFN) gamma, which plays an indirect role in brain plasticity and synapse formation. Changes in the microbiota can alter brain signaling, inducing some ASD behaviors by changing IFN-gamma concentrations. Some species of Bifidobacterium produce GABA, the concentrations of which have been found to be low in children with ASD. GABAs are closely related to the metabolism of glutamate, which is the main excitatory neurotransmitter in the brain. GABA/glutamate abnormalities could play an important role in the pathology of children with ASD. Likewise, alterations have been found in the metabolism of tryptophan (Trp), a precursor of serotonin, a neurotransmitter involved in almost all behaviors, including appetite, sleep, emotions, and cognitive and social skills. Lower levels of glutathione, homocysteine, methionine, and S-adenosylmethionine have also been observed in children with ASD compared to neurotypical children. Some of these are involved in sulfur metabolism and methylation, which contribute to the reduction of oxidative stress, cellular detoxification and excretion of heavy metals. Amino acid dysregulation in children with ASD could therefore be another mechanism underlying the development and severity of autistic symptomatology.
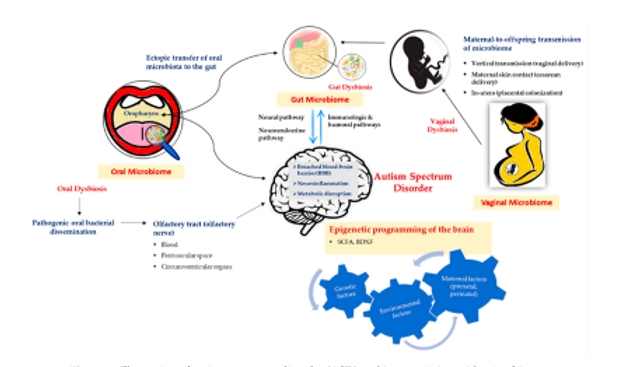
The figure below illustrates the possible mechanism involved in the microbiome-brain interaction in the context of the ASD disorder. Neural, neuroendocrine, and immunological and humoral pathways are the potential mediators in bidirectional communication in this axis. The maternal contribution is significant in determining early intestinal colonization in the offspring while, in general, environmental factors that significantly alter the maternal microbiome during the prenatal and perinatal periods influence the microbial composition of the offspring.
Another possible exchange pathway in the gut-brain axis is thought to be mediated by the oropharynx, which plays an important role in the pathology of ASD.
Some research indicates that malnutrition due to excess (overweight/obesity) is more frequent in children with ASD. Food selectivity and the characteristic eating habits of these children appear to result in a deficiency of some micronutrients, with significantly lower intakes of protein, calcium, phosphorus, selenium, vitamin D, thiamin, riboflavin, and vitamin B12, and higher intakes of fatty acids. polyunsaturated fats and vitamin E, lower intake of omega-3 and higher intake of fruits and vegetables, compared with neurotypical children.
In sum, ASD is one of the neurodevelopmental disorders that has been most explored in relation to the gut microbiome. The use of microbial knowledge promises a more integrative perspective in understanding this disorder, although it is an emerging field of research. Microbiota-based interventions include: diet, prebiotics, probiotics, antibiotics, and fecal microbial transplantation (FMT). Early administration of probiotics can reduce the risk of developing ASD. Clinical trials with probiotics containing Lactobacillus and Bifidobacterium strains have reported improvements in mood, anxiety, sleep quality, and depression. TMF also appears to enhance bacterial diversity by significantly increasing the abundance of Bifidobacterium, Desulfovibrio, and Prevotella persisting at 24 months.
Diet is probably the most powerful tool for microbiota modulation, and learning how to use it to generate a healthy microbiota should be a priority. It is important to prevent risk factors such as obesity, low weight, vitamin deficiencies; as well as detect hypo or hyperthyroidism and dyslipidemia, since its treatment would contribute to improving the quality of life of the patient and the family. To the extent that we better understand diet-host-microbiota interactions, we will be able to capitalize on true preventive and personalized medicine.
It was first suggested in the 1980s that eating foods containing proteins such as gluten and casein could cause autism-like symptoms by altering brain function. Casein is one of the proteins that most often causes immune reactions in children. In one study, children with ASD who follow a gluten- and casein-free diet (GFCF) have less tumor necrosis factor-α (TNF-α) than those who do not follow the diet. In children with ASD, it has been reported that incompletely digested peptides cross the hyper-permeable intestinal mucosa and through the blood, crossing the blood-brain barrier, reaching the central nervous system and having negative effects on attention, maturation, social communication and learning.
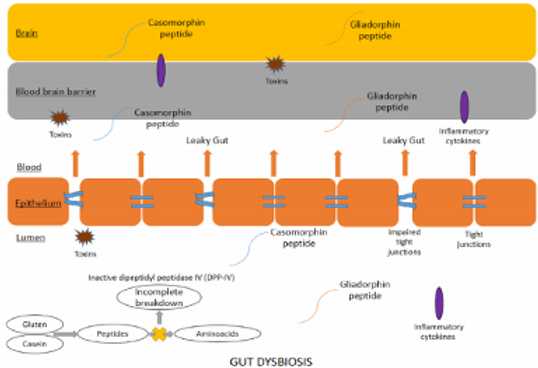
For 50 years the Autism Research Institute in California and in the same line LINCA (League of Nutritional Intervention in Autism and Attention Deficit Hyperactivity Disorder-ADHD) since 2000 in Mexico, promote therapeutic testing in children with ASD and ADHD of the gluten, casein and soy exclusion diet, low in sugars, as well as the supplementation of deficient nutrients, since it continues to report improvement in gastrointestinal, neurological, behavioral symptoms as well as sleep disorders and hyperactivity in some patients . The difficulties in generating solid scientific evidence in this approach lie not only in the lack of funds for this type of studies, which generally end up covering small populations of children, but also in the heterogeneity of the Disorder, as well as its therapeutic approach from multiple disciplines , which makes the study more complex when comparing with control cases. The meta-analyses that evaluate the effect of diet, to date, continue to conclude that although there are indications that this dietary approach could be useful, more studies are needed to achieve a sufficient level of evidence to advise all patients.
AUTHORS OF THE ARTICLE
Dr. Ileana C. Carzoglio Algazi, MSc
· Doctor of Medicine, graduated from UDELAR 2005.
· Master in Clinical Nutrition graduated from the Catholic University of Uruguay.
· Guest teacher at UCUDAL.
· Specialized in Obesity, member of the Uruguayan Society for the Study of Obesity (SUEO), founding member of the Uruguayan Society of Bariatric and Metabolic Surgery (SUCBM).
· Specialized in Gut Microbiota. Member of the Ibero-American Society of Microbiota, Probiotics and Prebiotics; the Uruguayan Nutrition Society (SUNUT); the Uruguayan Society of Psycho-Neuro-Immuno-Endocrinology (SUPNIE).
· LINCA Certification (League of Nutritional Intervention in Autism and ADHD - Mexico) in biomedical approach in ASD (2017 and 2020)
· Multidisciplinary approach to patients with pathologies involving the Microbiota.
Lic. Nta. Paula Mendive Dubourdieu, MSc
· Degree in Nutrition from the Complutense University of Madrid.
· Master in Genetic, Nutritional and Environmental Conditions of Growth and Development from the University of Cantabria.
· PhD in Biomedical Sciences at the Catholic University of Buenos Aires.
· Diploma Integration in Health from Psycho Neuro Immuno Endocrinology, UCU.
· Professor at the School of Nutrition at UDELAR and UCUDAL.
LINCA Certified (League of Nutritional Intervention in Autism and ADHD – Mexico)
· Certification in the Role of the Gut Microbiota in Human Health, INTA University of Chile.
· Nutritional approach in multidisciplinary teams for patients with pathologies or alterations that involve the intestinal microbiota.
Qin J. et al. A human gut microbial gene catalog established by metagenomic sequencing. Nature 2010 (464):59–65. https://doi.org/10.1038/nature08821. Ahlawat S et al. Gut–organ axis: a microbial outreach and networking. Lett Appl Microbiol. 2020. https://doi.org/10.1111/lam.13333. Ratsika, Anna et al. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients vol. 13.2 423. 28 Jan. 2021, doi:10.3390/nu13020423 Iglesias-Vázquez, Lucía et al. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients vol. 12.3792. 17 Mar. 2020, doi:10.3390/nu12030792
We will reply as soon as possible.
30 N Gould Ste N, Sheridan, WY 82801, USA
Our groups are the ideal platform to learn and share your scientific concerns about neurodevelopment issues
*Our purpose is informational only, it is not intended to be a substitute for medical advice, diagnosis or treatment.
We are working on our website. For any queries, you can contact our customer service team at atencionalcliente@eneviahealth.com