CONTACT
We will reply as soon as possible.
Enevia Health, LLC
30 N Gould Ste N, Sheridan, WY 82801, USA
The intestine is considered the main immune organ of the adult, since it is home to most of the body's immunocompetent cells, in addition to the fact that it has been proven that it is also the second in number of neurons. This discovery gave rise to the name gut-brain axis.
The microbiota is the set of microorganisms that inhabit the human body, and the composition of this group is not coincidental; In turn, the term microbiome encompasses the genomes of all the microorganisms in a given environment. This complex ecosystem is characterized by a complicated network of positive and negative interrelationships that significantly impact the health of the host.
Microorganisms coexist in various areas of the human body, from the skin, through the mouth, upper respiratory tract, ear canal and vagina. However, 90% of all microorganisms colonize the initial sections of the small and large intestine. Many studies indicate that the number of microorganisms that inhabit a macroorganism exceeds ten times the number of its cells.
The microbiota is made up not only of bacteria, but also of fungi, viruses and some protists. An essential element of the intestinal microbiota are also microorganisms that belong to a separate kingdom of living organisms, archaebacteria. These microorganisms are found in a wide variety, often in extreme environments, under strictly anaerobic conditions, obtaining energy from the transformation of inorganic or simple organic compounds.
Due to variable pH conditions in different parts of the gastrointestinal (GI) tract, oxygen conditions, access to nutrients and also intestinal peristalsis vary, and sections of the GI are colonized by specific microorganisms in consequence of these factors. Any alteration in the composition and quality of individual microbial communities can have serious health consequences. A characteristic and quite clear example that illustrates these disorders is SIBO.
Small intestinal bacterial overgrowth (SIBO) occurs when there is an abnormal increase in the entire bacterial population in the small intestine, particularly types of bacteria that are not commonly found in that part of the tube. digestive.
Among the functions of the intestinal microbiome we can mention:
It should be noted that the intestinal microbiota significantly affects the activity and functioning of the immune system, it is an immunomodulator, that is, it regulates cytokine levels through interaction with the lymphatic tissue of the digestive tract and is considered the largest lymphatic organ in the body. human.
Some studies have shown in cases of major depressive disorder (MDD), schizophrenia (SCZ) and bipolar disorder (BD), after comparing them with controls, differences were observed in the composition (β-diversity) of the microbiota. Such studies include experiments with germ-free (GF) mice raised in completely sterile conditions, without intestinal microbiota, investigations with the use of probiotics and antibiotic therapy, studies on the use of fecal microbiota transplantation (FMT) and the use of infectious investigations.
The connection between the IM and the central nervous system (CNS) is through bidirectional communication and begins during intrauterine life, it is affected by many intrinsic and extrinsic factors, such as vaginal birth/cesarean section, lifestyle habits, living arrangements (urban or rural), food and medication intake, and the host's circadian clock.
The critical factor for the formation of the microbiota that affects the colonization of the gastrointestinal tract by newborns is the mode of delivery. During the last decades, despite the lack of medical recommendations, the number of cesarean sections (CS) worldwide has increased.
Studies have shown that the composition of the gut microbiota in vaginal deliveries (VD) appears to be similar to that of the maternal vaginal microbiota: it predominates Lactobacillus, followed by Senathia spp. and Prevotella, most of which are anaerobic bacteria.
CS leads to an imbalance of the infant gut microbiota and a decrease in diversity. Due to the lack of opportunities to encounter the maternal vaginal microbiota, but a broad encounter with the hospital environment and the mother's skin are what constitute their first contact. As a result, pathogens (Enterococcus, Enterobacter and Klebsiella) from the hospital environment have been found in their intestines.
Studies have observed that the intestinal microbiota of the infant released by CS contains a lower abundance of Bifidobacteria, Bacteroides, Staphylococcus, Corynebacterium and a Propionibacterium spp. A greater amount of Lactobacillus, Prevotella, Sneathia spp. and Clostridium difficile compared to VD children. It is worth mentioning that a high abundance of C. difficile could cause dysbiosis and an increased risk of developing obesity.
There is a bidirectional connection between the intestine and the brain based on metabolic, endocrine, neuronal and immunological pathways. It includes the vagus nerve, the hypothalamic-pituitary-adrenal (HPA) axis, the production of bacterial metabolites, immune mediators, and entero-endocrine signaling.
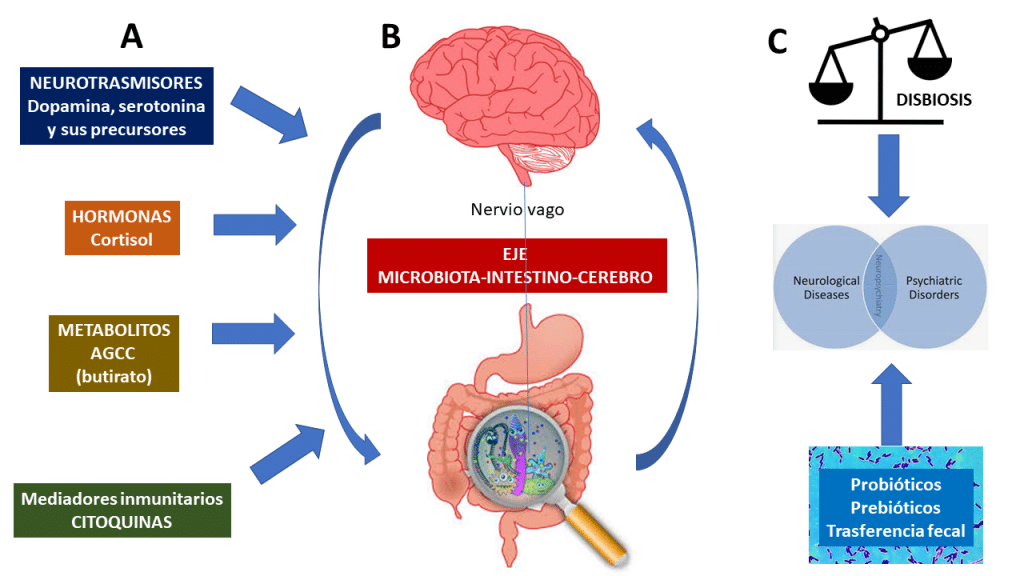
The brain-gut axis communicates through the autonomic nervous system (AUN), mainly the vagus nerve. Research has revealed that 90% of impulses within the brain-gut axis are transmitted centripetally, that is, from the intestines to the brain, and only 10% centrally. According to many observations, it has been proven that after vagotomy, the intestines continue to function correctly.
An important pathway to take into account is that intestinal bacteria produce numerous substances such as γ-aminobutyric acid (GABA) (Lactobacillus spp., Bifidobacterium spp.), acetylcholine (Lactobacillus spp.), serotonin (Escherichia spp., Candida spp., Enterococcus spp.), dopamine (Bacillus spp.) or norepinephrine (Bacillus spp., Saccharomyces spp.).
These substances are involved not only in communication within the intestinal microflora, but also in the systemic and peripheral effects that support proper brain function.
The gut microbiota also influences the level and metabolism of glutamic acid (glutamate), one of the main CNS stimulants. Glutamic acid is synthesized in glial cells. The most important receptor of the glutamatergic system is the N-methyl-d-aspartate (NMDA) receptor [108]. Under physiological conditions, glutamic acid is the basis of learning and memory.
Therefore, it has been widely applied to treat memory disorders and nervous exhaustion. But we must emphasize that everything in excess is harmful, and the hyperproduction of this acid causes overactivation of glutamatergic receptors and, consequently, damages neurons. Both glutamine and glutamic acid are converted to GABA, the major inhibitory neurotransmitter, in other metabolic pathways.
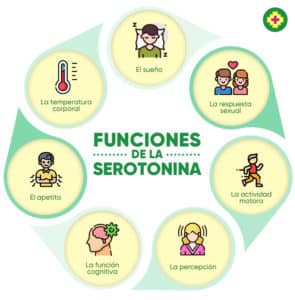
Another important product to mention is the synthesis of serotonin, commonly known as the "happiness hormone." Serotonin deficiency in the CNS is one of the factors that cause depression, sadness, apathy and anxiety. In the body, serotonin is produced in the digestive tract, nervous system, and immune system.
In the gastrointestinal tract, up to 95% of serotonin is produced, which is produced by the enterochromatophilic cells (ECC) of the mucosa, the microorganisms that are part of the intestinal microbiota and the neurons of the nervous plexuses of the submucosal layers. and muscles of the intestine. Of the other 5%, a 3% of serotonin is produced in thrombocytes and 2% in the pineal gland.
The main function of serotonin in the nervous system is neurotransmission, where serotonergic neurons play a crucial role in the regulation of pain, sleep, mood changes and also in memory processes. Studies also showed that the intestinal bacteria Bifidobacterium infantis affects the level and metabolism of tryptophan, thereby increasing its level in the body.
Another factor to take into account that leads to the systemic inflammatory response is increased intestinal permeability, also known as leaky gut syndrome (LGS).
So alterations of the intestinal microbiota within the gastrointestinal tract, damage to enterocytes, a weakening of the tight connections between enterocytes, but also stress with increases in cortisol levels, all of this plays a particularly important role in the pathophysiology of depression.
As a result of the development of this syndrome, the translocation of gram-negative bacteria containing lipopolysaccharides (LPS) causes overactivation of the immune system.
Which causes an increase in production and therefore in the concentration of proinflammatory cytokines, the excess of which is destructive for host cells, including CNS cells. All of this leads to general inflammation of the body, which is why the hypotheses are supported that these effects influence the development of affective disorders.
One of the most important and dangerous neurodevelopmental diseases related to the composition of the gastrointestinal microbiota is autism spectrum disorder (ASD). According to reports from the CDC's Autism and Developmental Disabilities Monitoring Network (ADDM), approximately 1 in 44 children in the United States has been diagnosed with ASD.
Some studies indicated genetic factors, other studies gastrointestinal abnormalities, inflammation or other individual and external factors (for example, pre- and postnatal exposure, stress, gastrointestinal microbiota or diet), although none of them are able to completely elucidate this disorder.
A common neurodevelopmental disorder is attention deficit hyperactivity disorder (ADHD), which affects 6 million children ages 3 to 17. Clinically, it manifests itself with certain patterns that include difficulty maintaining attention and sudden and unexpected behavior.
The dopamine receptor genes DRD4 and DRD5 and dopamine and serotonin transmitters are considered the main etiological factors of this disease.
But there is increasing evidence indicating a connection with the microbiome-gut-brain axis. In microbiome studies the results revealed differences in the taxa of microbiota composition, where in cases of ADHD, the phylum Actinobacteria was more abundant (e.g., Bifidobacterium), while the abundance of Firmicutes decreased.
Interestingly, the same study revealed that the microbiome of ADHD cases shows a more notable ability to produce cyclohexadienyl dehydratase (CDT), which is involved in the synthesis of the dopamine precursor (phenylalanine).
Intake of omega-3 polyunsaturated fatty acids is also significant, particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), as both are essential for proper membrane fluidity, neurotransmission, and brain function. receivers.
In a study based on male animal models of ADHD, nutritious individuals with a diet enriched in omega-3 polyunsaturated fatty acids caused a decrease in impulsivity and an improvement in concentration.
Schizophrenia is a multifactorial disorder that involves emotional, occupational, and cognitive deficits. Due to cardiovascular, metabolic and infectious diseases, adults are at risk of premature death from this disease. For people in the United States, the average potential life lost is estimated to be 28.5 years among those with schizophrenia.
The studies demonstrated three different dimensions determined as positive symptoms (hallucinations and loss of contact with reality), negative symptoms (decreased motivation and withdrawal), and cognitive weakness (limited efficiency compared to controls).
The increase in dopaminergic transmission in the mesolimbic part and the inhibition of glutamatergic transmission play an important role in the pathogenesis of positive symptoms, so the non-regulation or hyperregulation of this system by the gut-brain axis can affect the prevalence of positive symptoms.
Another serious mental illness is bipolar disorder (BD), a chronic, relapsing illness characterized by the recurrence of hypermanic episodes or subsequent depressive episodes, with some symptoms similar to those of schizophrenia.
Globally, it was estimated that between 2017 and 2019, 46 million people suffered from bipolar disorder, with New Zealanders being the most significant percentage.
The modification of the composition of the intestinal microbiota of patients is related to dysbiosis and disease progression.
According to studies that considered the diversity of the gut microbiome, a higher amount of Coriobacteriaceae was associated with an elevated cholesterol level, and a higher level of Lactobacilli contributes to the development of obesity.
The low amount of Faecalibacterium, a native intestinal bacteria, could also be correlated with the disease.
In patients diagnosed with bipolar disorder, the number of Clostridiaceae involved in the fermentation of carbohydrates leading to the production of short-chain fatty acids was four times lower than in the control group.
The gut microbiota is a modifiable target with the potential for epigenetic modification and could therefore be used to treat and improve symptoms of psychiatric disorders.
The gut-brain microbiota axis can be modified with certain prebiotics, that is, with a modification of the diet with foods rich in indigestible fiber, probiotics (live bacteria), antibiotics to control the overgrowth of certain colonies, synbiotics (combinations of prebiotics and probiotics ), postbiotics (bacterial fermentation products such as short-chain fatty acids) and fecal microbiota transplantation (FMT).
All of these approaches could be considered as potential psychobiotics, as they are suggested to improve mental health through their microbiota-modifying properties.
One of the main determinants of the composition of the intestinal microbiota are prebiotics and diet. Diets of animal and plant origin cause drastic changes in the intestinal microbiota in a matter of days.
Certain dietary styles, such as the Mediterranean diet, which has a high content of plant-based foods and fiber that promote the growth of beneficial bacteria.
Some dietary supplements, such as omega-3 fatty acids, are used in the treatment of depressive disorders, but most dietary supplements still lack scientific evidence. Additionally, probiotic dietary supplements have been widely tested as a complementary treatment for psychiatric disorders.
So thanks to all these findings and with future studies, it is likely that the area of nutrition and intestinal health will become an important component in the biopsychosocial treatment model in psychiatry, pediatrics and general medicine.
Therefore, the evolving field of nutritional psychiatry must be integrated into clinical practice to treat and prevent psychiatric disorders as well as metabolic comorbidities.
Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry – PMC (nih.gov)
The Microbiota-Gut-Brain Axis | Physiological Reviews (physiology.org)
If you are interested in this area, visit our website through www.eneviahealth.com, or we leave you the link to the previous articles already published on our blog.
We will reply as soon as possible.
30 N Gould Ste N, Sheridan, WY 82801, USA
Our groups are the ideal platform to learn and share your scientific concerns about neurodevelopment issues
*Our purpose is informational only, it is not intended to be a substitute for medical advice, diagnosis or treatment.
We are working on our website. For any queries, you can contact our customer service team at atencionalcliente@eneviahealth.com