CONTACTO
Responderemos lo antes posible.
Enevia Health, LLC
30 N Gould Ste N, Sheridan, WY 82801,
USA
By Natalia Mármol Cofounder of Enevia Health
In this interview we are going to be talking about a compound that I’m sure many of you have heard of either by the name of OSR/EMERAMIDE or NBMI. This compound is a heavy metal chelator with amazing properties that has the AUTISM community very interested in. To talk about it we have invited Dr. Haley Boyd, the person that invented this compound.
1. Doctor Haley, could you please explain what OSR is?
OSR or Oxidative Stress Relief is now called emeramide as it is being studied to obtain marketing approval as an FDA/EMA approved drug to treat mercury toxicity. In scientific articles it is called NBMI which is the abbreviation of its complex scientific name.
2. What were you pursuing when you formulated OSR?
The initial development of OSR was based on several aspects of my research life but most important was the belief that I had that the autism increase in the USA correlating with the CDC-USA mandated vaccine program for infants using thimerosal preserved vaccines was enhanced by exposure to this mercury containing preservative. I thought this primarily because of a publication from a medical group in Toronto (Canada) reporting that infants with umbilical cord infections treated with topical application of a commonly used thimerosal containing antiseptic resulted in the death of 10 infants from mercury toxicity.
Apparently, infants cannot handle exposure to thimerosal as well as adolescents and it builds up in their tissues to very toxic levels. The FDA subsequently banned the sale of topical antiseptics containing thimerosal after this report, but the CDC still thought it should be used in preserving vaccines for infants. This led to the decrease in the preservation of infant vaccines with thimerosal in the USA but it is not apparently banned even today.
3. Did you manage to achieve it?
I had NIH funding to study the nucleotide binding proteins of Alzheimer’s Diseased (AD) brain as we had published results showing major differences using bioprobes I had invented earlier. Following this lead we published data showing that mercury, and only mercury, when added to normal brain tissues could easily replicate the abnormal microtubulin properties found only in Alzheimer’s Diseased brain tissues. I was therefore very interested in any compound that could bind mercury (hg2+) and remove or reduce its toxicity.
I was chair of the department of chemistry at this time and hired an assistant professor interested in developing treatments for the heavy metal toxicity of coal mining water. I sat in on his graduate student presentations on possible chelators to treat such water. One compound that was studied primarily failed because it was not water soluble but had very high affinity for several toxic metals was of great interest to me and this was osr (AKA NBMI, emeramide).
What I had was an opinion that current chelators used in medicine (DMSA, DMPS, EDTA, etc.) were not effective because they were charged, hydrophilic compounds that did not effectively cross normal cell biomembranes nor cross the blood-brain barrier to get into the central nervous system. Also, those designed and used for mercury chelation were not true chemical chelators as the two thiols present in each are on adjacent carbons and to close together to allow mercury to fit sterically between them. So they form sandwich complexes with mercury which are weaker bonds. {this is a complex area with a lot of chemistry involved and may not make for a good interview for laypersons or even mds.}

4. What are the qualities of OSR? Could you explain them, please?
What is the comparison between the chelators that are frequently used and OSR?
The normally used mercury chelators DMPS and DMSA are charged and do not effectively leave the blood to enter any cells. They are rapidly cleared within 4-8 hours from the blood into the urine with or without metals bound. The only toxic metals that they remove are those found inside the arterial walls, they do not reach the intracellular toxic metals where most are located and causing toxic effects.


5. It was sold in the USA during over 2 years, about 3.000.000 packages were sold as a dietary supplement, with no side effects reported, what happened? Why did you stop selling it?
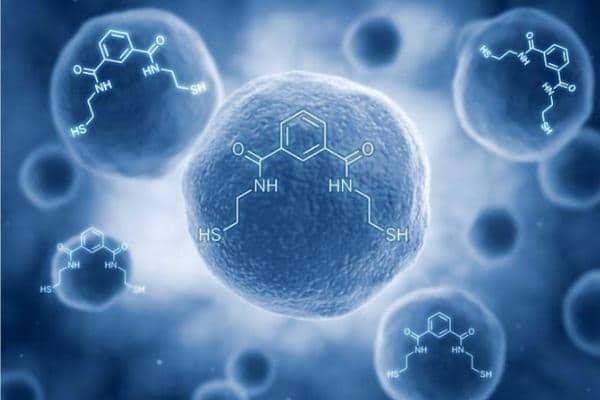
I introduced OSR as a food supplement antioxidant at that time under the FDA ruling that a food supplement could be claimed by any naturally occurring product or the combination of any two naturally occurring substances. OSR was the combination of dihydroxylbenzoate (found in several tart berries) and cysteamine (found in meat and on the terminal end of coenzyme-a). Benzoates are commonly considered a gras (generally recognized as safe) compound and cysteamine is a breakdown product of cysteine (it is the decarboxylated amino acid cysteine). I initially developed OSR because it was a structure that had two important structural features that allowed it to: (1) bind very tightly toxic metals attracted to thiols and (2) was uncharged and capable of passing through biomembranes to reach intracellular cytosols.

We had tested the OSR compound and knew it bound several toxic metals very tightly, we followed this up with several tests to show OSR ingestion or injection cause very low, if any, toxic induced effects. We then tested OSR for ORAC (Oxygen Radical Absorbance Capacity) and found it to be excellent in this regard. We sent it to a commercial ORAC testing company and confirmed this property, there would be few, if any, antioxidants that could be taken orally and match OSR’s ORAC score. Knowing autistic children were suffering from oxidative stress and many were being treated with ineffective antioxidants we thought OSR would be a good approach, OSR was safe, entered all the tissues of the body, was a powerful antioxidant and likely to chelate any toxic metals found in the body eliminating their toxicity such as binding the unbound fe3+ which donates an electron in the production of hydroxyl free radicals in the fenton reaction. After submitting Osr as a food supplement to the FDA we gave samples to medical doctors who had autistic children for them to use and review. This led to immediate encouragement when some mds reported that the porphyrin profile of their children returned to normal. We then made OSR commercially available as a dietary antioxidant.
*** Further clarification from Dr Haley on why they stopped marketing OSR in The USA:
The short answer is that the FDA after two years of OSR on the market with no “OSR related adverse effects” reported either to the EmeraMed OSR adverse effects site nor to the FDA sent us a letter that said since OSR was being used to treat a medical condition it had to be considered an unapproved drug and not a food supplement.
The medical condition was autism, as noted in reports from blog sites where parents of autistic children were reporting major health improvements in their children on taking OSR. I don’t know who informed the FDA of these sites but these sites were not associated directly with EmeraMed and our company did not make such claims as we knew this would lead to FDA shutting us down.
However, the FDA in their desist letter made their position clear, any compound sold that had a positive effect on any illness or disease had to be classified as a drug and had to have FDA approval. Since we did not have FDA drug approval for OSR the FDA sent agents to our company site daily for a week to ensure we were ending the shipping of OSR to MDs and dentists and therefore to parents of autistic children.
I contacted an experienced lawyer to deal with the issue and he told me that if I hired him, he was confident that he could win my case. However, he also told me that in his opinion, it would cost over a million dollars and that in the end he thought based on his experience that the FDA would quickly confiscate all my laboratory and computer equipment and bring other charges against EmeraMed and affiliates that helped us produce the drug. He recommended that I take the OSR through drug approval as it was very safe and effective and obviously needed. So that is what I did.
Due to our research we prepared and applied Orphan Drug Requests (ODRs) for both the EMA and FDA and both were accepted. These ODRs were for treatment of toxic heavy metal treatment primarily based on prevention and treatment of mercury toxicity.
We currently have OSR (as Emeramide) submitted to the FDA as a drug to treat mercury toxicity. We have met with FDA committees twice to discuss what is further needed to produce an acceptable New Drug Application (NDA) that could lead to obtaining marketing authority. The FDA has made statements to date agreeing that Emeramide (OSR) is safe and effective based on our animal studies. They also agreed that Emeramide is proven safe for human use due to the two years of marketing and 5 human Phase I/II trials conducted with no significant drug related adverse effects.
However, the FDA, who at first recommended that Emeramide be approved under the FDA’s animal rule, changed their minds. The FDA’s new position is that EmeraMed would have to do Phase II and III human trials to prove efficacy in humans.
However, when we have asked the FDA to identify a mercury toxic human population in the USA that could serve as testing subjects for such Phase III studies they have not yet responded. It is rare in the USA for a MD to suggest that mercury toxicity is involved in any causal way for any illness including autism. Dentists who have made comments that dental amalgam expose patients to toxic levels of mercury have had state dental board action taken against them.
MDs in the USA mostly avoid openly diagnosing mercury exposure as the cause of any illness. I have not heard of any USA clinic that treats mercury toxicity and I have searched diligently for one. I sincerely think our FDA does not consider mercury toxicity in the USA as a medical concern, even though they have taken a stand on the FDA website that dental amalgams are not safe for certain citizens that represent a majority of Americans.
I would point out that our FDA is not very proactive in drug development based on need. For example, not one FDA person has ever contacted me about the very positive claims being made on blog sites by parents of autistic children indicating that the use of OSR gave the most promising positive effects on the treatment of autistic children. This is amazing since the autism increase after the initiation of the CDC mandated vaccine program in about 1988 has been presented as a medical problem, and it apparently still is in many countries. The FDA/CDC recommendation to remove thimerosal (ethylmercury) as a preservative from infant vaccines shows these agencies know of the parental concern of such preserved vaccines.
Finally, I think the safety of infant vaccines is different in each country. Thimerosal vaccines are not outlawed in the USA, it is just that thimerosal free infant vaccines are available. It is likely that each nation has addressed the safety of thimerosal containing vaccines differently and this needs to be checked out in each country.

6. CURING AUTISM… Let’s talk about the autistic families that tried OSR, we know that heavy metal toxicity is a big problem in this community. Children that are not able to detoxify properly accumulate these metals causing an array of symptoms.
What these parents need to understand is that the oxidative stress in their children, since it starts at a very early age, is most likely caused by a toxin that induces the displacement of fe3+ from normal tissue leading to unbound iron and hydroxyl free radical formation and oxidative stress. Some autistic children will be more susceptible due to their genetics.
7. What did autistic families report to you after taking OSR?
8. TESTING OSR. We know that the excretion of OSR is through the stool, which makes stool testing the obvious choice, would any of the metals excrete by the urine?
Once OSR binds a cation with a +2 charge the complex is neutral and not readily excreted by the kidneys into the urine. That is the OSR-Hg complex formed inside a cell has to be modified by the body’s p-450 detox system to attach a charged water soluble moiety to the uncharged OSR-Hg complex to make it water soluble and excretable. In the phase I of the p-450 detox system a class of enzymes called cyp enzymes oxidize insoluble (hydrophobic) compounds setting them up for attachments in the p-450 phase II modification where to the oxidized site added by the cyp oxidation reacts with a phase II enzyme and the charged water soluble component is attached. In the case of OSR-Hg this appears to be glutathione attached by the p-450 phase II enzyme gst (gluthione-s-transferase) producing glutathione-OSR-Hg complex that is soluble and excretable into the plasma and placed in the bile through the biliary transport system and then into the fecal material. This is how Hg2+ is removed as the glutathione-Hg-glutathione complex as about 80-90% of mercury is excreted by healthy humans in the fecal material also.
Don’t miss the interview on Enevia Health’s YouTuBe channel
OTHER QUESTIONS:
* How often would you test?
This should be determined by the treating md as all cases are different.
* Does OSR depletes minerals?
Emeramed has never seen depletion in our human phase I and II studies. Nor has it occurred in test animals unless exceptionally high doses are given for long times.
* Are there any supplements that should or be recommended to take when taking OSR?
Yes, a supplement for molybdenum and to insure adequate zinc levels would be reasonable.
* Taking OSR, Is it better with an empty stomach or with food? (with coconut oil)?:
It appears as if OSR is slightly better absorbed with a fatty meal.
* Morning or night? no difference in absorbance.
* How does OSR interact with stomach acid?
it doesn’t appear to interact a lot.
* Can it increase/decrease Stomach pH?
Not chemically as the pH for protons on the OSR-thiols to be released has to reach greater than 10.0
* Do you know OSR where it gets absorbed mostly?
OSR peaks in the plasma at about 2-3 hours after ingestion. OSR enters the different organ from the plasma and it does not appear to leave organs of high OSR content (the liver and kidneys) and enter into other tissues. This is with or without mercury present in the tissues.
* It’s important not to take minerals together with OSR? How far apart should they be taken to be safe?
It is quite safe to take minerals while taking OSR but there should be time between the two ingestions just to be safe. I would suggest a minimum of 5-6 hours between the two.
* Do you think taking OSR can increase adrenal stress? Is it recommended to support the adrenals? Do you remember any ASD family talking about it?
I have no comment on this as we have not studied it.
* Anyone reporting, kids being more anxious or waking up in the middle of the night when taking OSR?
No, just the opposite, the reports were that the children slept better. However, to my knowledge this has not been adequately studied.
* When that happened, did you recommend the parents to stick with the doses and/or lower it and support adrenals?
I have never addressed this issue with a parent.

* Let’s talk about Sulphur sensitivities, probably the only contraindication of OSR is Sulphur sensitivity, lots of ASD people have that, would you then NOT recommend taking OSR? Is there any way that people that have this sensitivity can take OSR?
Yes, if their sensitivity is eliminated by molybdenum supplementation then taking OSR is helpful.
* When is it advised to take molybdenum?
As a regular supplement weeks before OSR treatment.
* What would be important for an ASD parent to have in consideration before giving OSR?
This should be decided by the treating md: Genetics, P450, levels of minerals, etc…
* What are the doses that ASD people took and saw better results?
Anywhere from 100 to 400 mg/day. Larger and older children should take larger doses, again this should be done under treating md advice.
* Is it better to begin with really small amounts (a tooth pick for example) and build up over time or is it better to go full dose?
I always recommend to go low and go slow but try to build up the dosage to an effective level within two weeks.
* What heavy metal won’t bind to OSR?
We can assure you that OSR does not bind Ca2+, Mg2+, K+ and Na + but we have not studied other metals to determine their binding. OSR will bind to every heavy metal that has an attraction for SULPHUR, which is almost all of them. Mercury (strong bond), uranium, led, free iron and copper.

* Storing, what is the best way to store OSR?
Best to keep it refrigerated, but it is reasonably stable at room temperature if kept sealed.
* For how long can it be kept?
We have established a 5 year shelf life for emeramide. We have also tested an 8-10 year old OSR that still appears good.
* Can you tell us what is the status of OSR in USA?
It is not FDA approved for any treatment and is available only through foreign chemical companies who have to claim it is not for human consumption. As you know, there is an active black market selling of OSR in the USA that our government does not attempt to prevent as far as i know. Emeramed does not sell OSR or emeramide.
* Is it possible to manufacture and sell OSR in Europe as a nutritional supplement?
I think now but I don’t really know. likely depends on the country.
* What happened to compassionate use in the EU?
Emeramed shut it down because it cost the company too much and did not help with regulatory approvals.
* When do you think you will get FDA approval to sell OSR again in The US?
I really don’t know. We are going to try but we are also trying to get approval in SouthAmerica and África. This is not a clear and easy business and is fraught with knock companies who do not make a proven pure and safe product. I am helping you because I want to keep the use of OSR as safe as possible and worry about the safety of the knock off products.
* The company that is creating OSR is based in Sweden. Why is it not a US based company?
Not true. Emeramed is based in Ireland with offices in Sweden and the USA. We have applied for and been awarded orphan drug development in the EU and USA.
* Does OSR bind to Mg transiently and can that be used to enhance absorption into the body and cell membranes?
I don’t have any indication that this occurs.
* Would it be possible to make OSR liposomal (IE, surround it with a Phosphatidylcholine bubble)?
Why, it is hydrophobic to start with and is readily absorbed in all organs of the body tested
* Is it possible the OSR binds to Free Iron then finds Free Mercury and loses its bond to the free Iron? Will this Happen for other heavy metals here, like would Mercury be able to kick outbound Lead and bound Uranium?
This is possible but very unlikely as the unbound mercury levels are incredibly low in the body.
* Some people need to take Molybdenum to tolerate OSR better, is it possible that the thiols on the OSR benzene ring degrade to sulphites? (hence why the need for Molybdenum)
The thiols on the benzene ring are only converted to sulfites on reaction with hydroxyl free radical in solution. If stored in a dry powder OSR thiols are stable for years.
* Mercury and heavy metals damage Heme and Cytochromes, after some use of OSR treatment and large reduction of Heavy Metals would be necessary to replenish and help the body build these cytochrome using Iron, Copper and (5-ALA – 5-Aminolevulinic Acid)
These could all be good co-treatments but need further investigation.
* If a child is sick (with a cold/virus) would you still give the OSR, we have heard you say in other interviews that OSR has antiviral properties, can you elaborate this further, please? Absolutely, we know that treatment with OSR raises the intracellular reduced glutathione levels in mammals and increased glutathione levels inhibit viral infection rates. This is why covid did not cause as many major problems in the younger set (higher glutathione levels) as it did in the aged set (much lower glutathione levels).

YouTube: https://www.youtube.com/c/EneviaHealthSL
Facebook: https://www.facebook.com/eneviahealth
Instagram: @enevia_health
Responderemos lo antes posible.
30 N Gould Ste N, Sheridan, WY 82801,
USA
Nuestros grupos son la plataforma ideal para aprender y compartir tus inquietudes científicas sobre los temas de neurodesarrollo
*Nuestro fin es informativo únicamente, no se pretende sustituir el asesoramiento, el diagnóstico o el tratamiento médico.
Estamos trabajando en nuestra web. Para cualquier consulta, puedes comunicarte con nuestro equipo de atención al cliente en atencionalcliente@eneviahealth.com